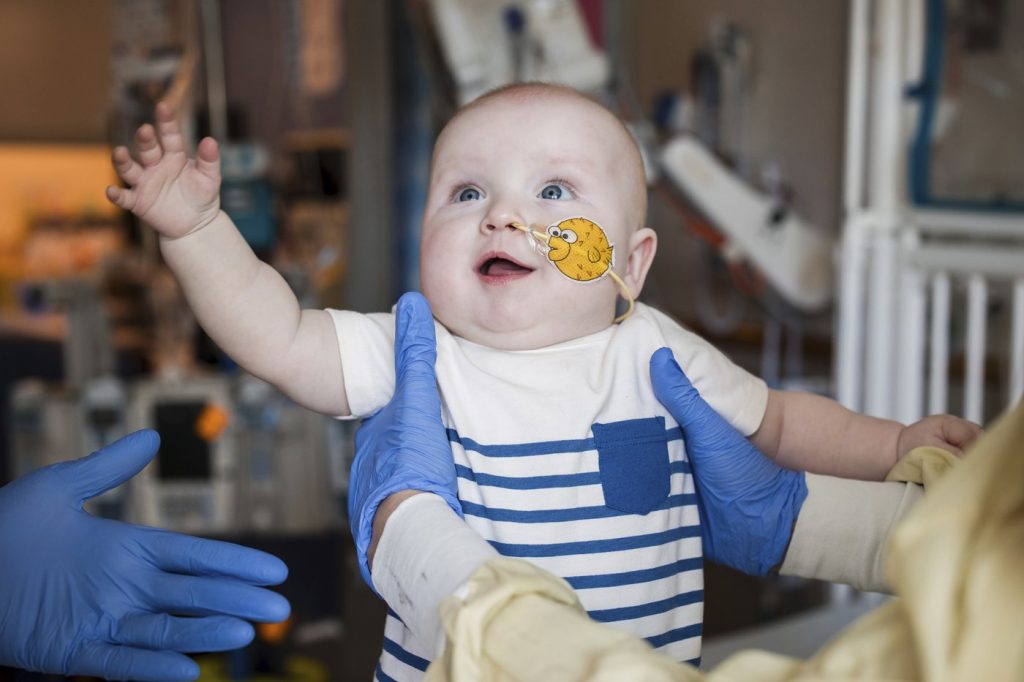
A baby born with a rare and dangerous genetic disease, named KJ Muldoon, is experiencing significant improvement after undergoing an experimental gene editing treatment specifically designed for him. Researchers recently shared KJ's case in a study published in the New England Journal of Medicine, highlighting his treatment as one of the first successful applications of this custom therapy, which aims to correct a crucial error in his genetic code responsible for a condition that kills half of those affected.
KJ, born in Clifton Heights, Pennsylvania, was diagnosed shortly after birth with severe CPS1 deficiency, a rare genetic disorder affecting approximately one in a million infants. This condition leads to a deficiency of an enzyme essential for ammonia removal from the body, causing potential toxicity. Though a liver transplant can be an option, KJ's parents, Kyle and Nicole Muldoon, both 34, faced the heart-wrenching decision about whether to pursue this invasive treatment or participate in an untested experimental therapy.
Determined to help their son, the Muldoons gathered information, prayed, and ultimately chose the experimental therapy. Within six months, researchers at Children’s Hospital of Philadelphia and Penn Medicine developed a gene therapy specifically targeting KJ's faulty gene. Utilizing the CRISPR gene editing technology, which was awarded a Nobel Prize in 2020, the medical team employed a novel technique called "base editing." Unlike earlier CRISPR methods that cut DNA strands, base editing allows researchers to flip the mutated DNA letter to the correct base, significantly reducing the risk of unintended genetic alterations.
KJ received his initial intravenous infusion of gene editing therapy in February 2025. This treatment was delivered using lipid nanoparticles that facilitate uptake by liver cells. Although the infusion day was filled with anticipation and excitement, KJ remained peacefully asleep during the procedure, as recalled by study author Dr. Rebecca Ahrens-Nicklas, an expert in gene therapy at CHOP. After follow-up doses in March and April, KJ demonstrated remarkable progress, such as a more normal eating regimen and increased resilience against common illnesses that can worsen his condition. Notably, his parents expressed joy at witnessing even the smallest milestones, such as him waving or rolling over.
Researchers are cautiously optimistic about KJ's results, reiterating that it has only been a few months since treatment and that continuous monitoring will be necessary for years to come. Dr. Ahrens-Nicklas noted that while the early signs are promising, there is still much to learn about the long-term effects of the gene therapy.
Moreover, the team emphasized the importance of developing personalized gene therapies for rare genetic disorders. Currently, gene therapies often focus on more common diseases primarily due to financial considerations; larger patient populations can lead to higher sales and recovery of development costs. However, Musunuru, one of the study's authors, highlighted that creating a custom treatment for KJ was manageable and not excessively costly, aligning closely with the financial burden of an average liver transplant.
As the field of gene therapy advances, researchers believe that better techniques and economies of scale will further drive down costs, making these personalized treatments more accessible in the future. Other experts, such as Carlos Moraes from the University of Miami, assert that breakthroughs like KJ's treatment could pave the way for more innovative approaches and knowledge transitions across scientific teams, indicating a promising future for the treatment of rare genetic disorders.