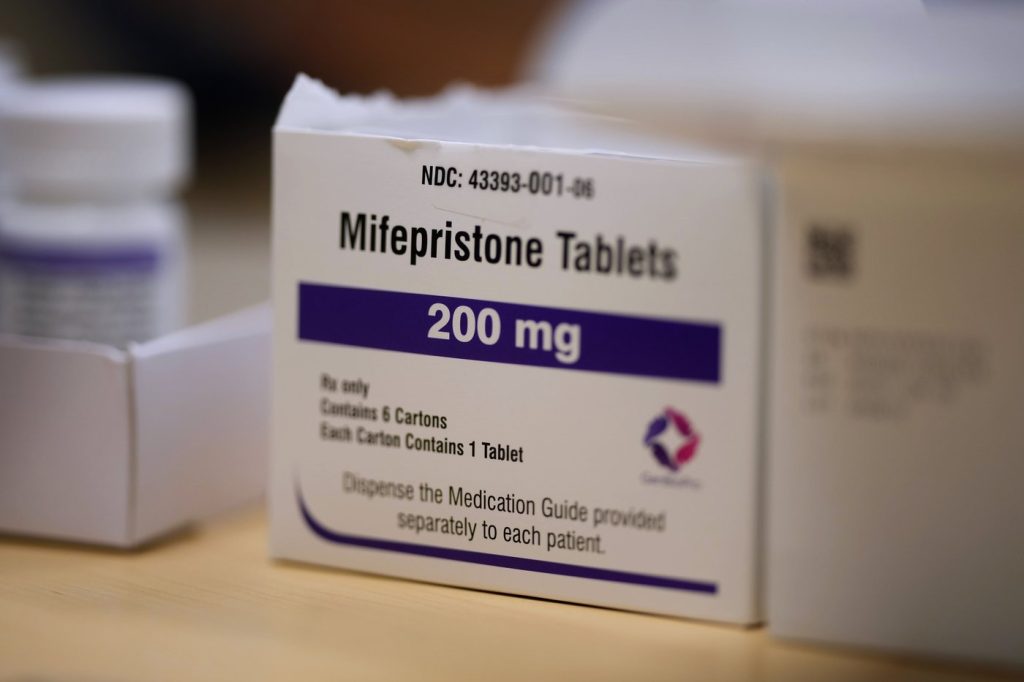
Arguments regarding groundbreaking legislation that designates two commonly used abortion-inducing medications, misoprostol and mifepristone, as "controlled dangerous substances" in Louisiana are set to be heard before a state judge on Thursday morning. This legal challenge, initiated in October, contends that the reclassification of these drugs could result in unnecessary and potentially life-threatening delays in medical treatment during emergencies.
The plaintiffs argue that the new law may impede access to critical, lifesaving treatments for individuals facing obstetrical emergencies. They assert that it could complicate the process for patients to obtain essential medications needed for their care. The plaintiffs are requesting the judge to issue a permanent injunction to halt the law, which is currently in effect.
Defendants, including Louisiana Attorney General Liz Murrill, are seeking to dismiss the lawsuit. Murrill expressed her anticipation to "vigorously defend this law" in court. This legislation, passed by Louisiana's GOP-dominated Legislature, represents a novel strategy in conservative efforts to limit access to abortion pills. Louisiana became the first state last year to elevate the classification of these medications, which is consequential as nearly two-thirds of abortions in the United States in 2023 were medication-based.
The impetus for this legislation has roots in anti-abortion advocacy and a Republican state senator's initiative aimed at preventing coerced abortions and restricting access to the drugs by allegedly nefarious individuals. The lawmaker cited a personal instance involving his sister, who was unknowingly given misoprostol by her husband, emphasizing that such cases warrant stricter controls. However, it is noted that similar incidents have not been reported in Louisiana and do not appear to be widespread.
Attorney General Murrill emphasized the legislature's firm stance on protecting life, stating that the reclassification aligns with their commitment against the distribution of controlled substances without a doctor's prescription. Previously, patients needed a prescription to access mifepristone and misoprostol, but these drugs were also readily available in medical settings for various use cases, including treating miscarriages, inducing labor, and stopping bleeding. Medical professionals noted that these medications were often stored in easily accessible locations within hospitals to ensure rapid access in emergencies.
The new law places mifepristone and misoprostol in the "Schedule IV drugs" category, similar to the opioid tramadol, which introduces additional storage requirements and procedural hurdles. Doctors who testified against the legislation indicated that the new regulations could lead to slower access to these medications in urgent situations, where timing is critical.
Furthermore, the heightened classification brings about harsher penalties. Individuals found in possession of these drugs without a valid prescription could face fines of up to $5,000 and imprisonment for one to five years. However, the law does offer protections for pregnant women who obtain these medications without a prescription for personal use.
The plaintiffs in the lawsuit include a physician, a pharmacist, the Birthmark Doula Collective (an organization specializing in pregnancy care), Nancy Davis (a woman who sought an abortion in Louisiana and went out of state after learning her fetus would not survive), and another woman who reported being denied treatment for a miscarriage at two emergency rooms.
The legal proceeding seeking a permanent injunction against the law is being conducted in the 19th Judicial District Court in Baton Rouge, with the Thursday hearing anticipated to address the state’s motion to dismiss the lawsuit.